More from News 12
0:10

Crews respond to SUV on fire on Route 1 in Avenel
2:05
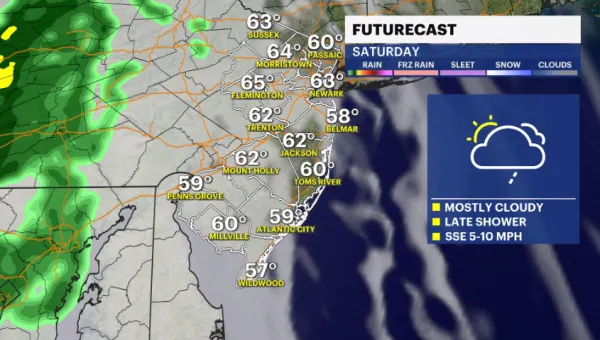
Temperatures dip overnight; Weekend to feature sun and clouds with some light rain
1:08
New Jersey man says his business and family are at risk if TikTok app is banned
1:43

Police: 2 people injured when tractor-trailer crashes into Cedar Grove pharmacy
0:26
Police: Man charged with wielding machete during road rage incident
0:14

Police: Hazlet man accused of setting multiple fires over span of 5 months
0:53

Jersey Proud: Ramapo College genealogy team solves 22-year-old cold case
2:02

MTA pushes back start date of congestion pricing two weeks to June 30
0:20

Burglary at Manchester Township bagel shop caught on video
1:53

Shop Mother’s Day Gifts – Exclusive Offers Up to 75% OFF!
3:03

Rutgers students remain on alert amid uptick of incidents near New Brunswick campus as 'Rutgers Day' nears
2:25

Middletown street renamed in honor of iconic New Jersey brothers Stevie and Billy Van Zandt
0:25

Services announced for US Rep. Donald Payne, Jr. who died at 65
1:09

Workers call out Gov. Murphy for saying there are 2 sides to casino smoking ban proposal
0:31

Police: Former Giants lineman Korey Cunningham, 28, found dead in Clifton home
1:21

SUV crashes through Dunkin' in Old Bridge
0:23

North Wildwood approved for emergency beach replenishment
0:52

Clothesline Project raises awareness of sexual assault survivors
0:42

Police: 3 women accused of stealing nearly $600 worth of merchandise from Target
2:17
